The act of synthesizing a molecule especially for a certain customer based on their specifications at their size is known as custom synthesis. It is one of the options available to pharmaceutical companies looking for active pharmaceutical ingredients or metabolites to start R&D projects.
Because many of these compounds are difficult to obtain or make, custom synthesis is important for pharmaceutical companies. Custom synthesis provides clients with the infrastructure they require to get their goods to market as quickly as possible.
Custom Synthesis is the best method for synthesizing:
The drug developers have opted to build the treatment based on their requirements; thus, they are employing bespoke synthesis in the drug development procedure. Now, let’s understand some in-depth information on drug development and biology services.
This is regarded as the most important function in medication development. Before beginning the formulations of new medicine, you must examine the age and condition of the components. The formulations that are being developed should be very effective at biochemical, cellular, and functional levels for the human body.
- Reagents for chemistry
- Catalysts and ligands are two types of ligands.
- Blocks of Construction
- Organic electronics materials
- Chemical solutions/standards
- Compounds like lead or drugs
- Service for Chemicals (Reaction screening, Special packaging, etc)
- Polymers
Benefits of Custom Synthesis:
There are several potential advantageswhen it comes to synthetic chemistry. We may have expertise in a certain area of chemistry or facilities that the pharmaceutical industry does not have. A major pharma company undoubtedly has its own in-house synthesis teams; nevertheless, they may be better served to do research and development work than simply manufacturing something that has already been produced.
Pay rates at pharmaceutical companies are, as one might assume, much higher than those at suppliers. And, if they have their own in-house resources, there may be peaks and troughs; when there are surges, they may not have the in-house resources to conduct the chemical, so they may seek assistance from external suppliers.
Scaling up a medicinal chemist’s technique is frequently impossible since it would be dangerous. For example, many medicinal chemists may do “all on board” reactions, in which they combine all of their agents in a pot and then heat it up.
If an exotherm heat is created in that process, it is generally manageable because it is on such a tiny scale. But you can’t do it on a huge scale because combining all of your molecules and generating a lot of heat may be really hazardous.
Custom Synthesis for Drug Development:
In today’s culture, drug developers play an essential role. Nowadays, most individuals are afflicted with different ailments as a result of their contemporary lifestyle. They all take their medications on a regular basis, as prescribed by their doctors.
As a result, medication developers are attempting to offer effective treatments that meet the needs of patients. Following the identification of early “hits,” a targeted library of comparable compounds will be produced to maximize interactions with the therapeutic target; this is the library synthesis process.
Custom synthesis refers to the exclusive synthesis of compounds on behalf of the client, i.e., you may order a specific molecule that is only produced on the scale, purity, and specifications or techniques you desire.
A Contract Research Organization (CRO) enables the outsourcing of a wide range of research activities, from the custom synthesis of small molecules to clinical trials of a new drug, thereby assisting in the transformation of the commercial research culture into an international, innovation-based industry. CROs, with their new epistemic abilities and powers, are both expressions of and drivers for changes in commercial research cultures.
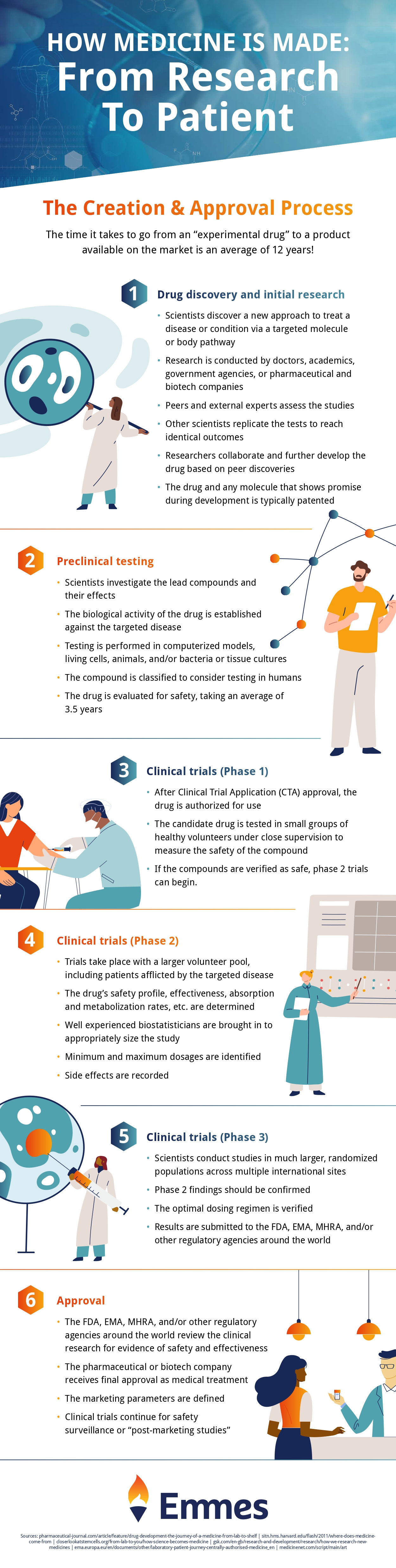
Before the treatment can be authorized by the FDA, drug makers must do years of testing and study. The long procedures that drug researchers must follow in order to obtain clearance for a certain treatment before it can be sold on the market. Drug development for chronic and hereditary diseases is a difficult challenge for drug developers and researchers.
CROs, on the other hand, primarily focus on two parts of the value chain: small-scale production of active components for novel medicines and the design and fundamental development of the manufacturing process.
As a result, macros are gaining traction, and the general public perceives pharmaceutical development outsourcing to primarily comprise duties such as lead identification, compound screening, lead optimization, or clinical trials. As a result, the market for contract firms providing bespoke synthesis and research services is far less than that of macros.
Is it true that custom synthesis and contract research are exclusively available to major pharmaceutical corporations?
This service is not focused on a single industry and does not appeal to a specific type of client. While certain CROs specialize in serving pharmaceutical companies, the nature of this service does not vary greatly across customer groups.
Because CROs help with the development of new compounds, anyone looking for new molecules is a possible client of these service firms. Outsourcing chemical contract research and synthesis projects is a fantastic option whether you are a small start-up biotech company, a polymer developer, a cosmetics specialist, or a large specialized chemical manufacturing company.
Bottom Line:
Healthy volunteers were used to testing the new medicines. The individual must be in the absorption phase of the medication until it is excreted. The medication developers must next determine if the introduced medicine is capable of treating that condition or not.
The following step requires them to introduce that specific medicine in various dose levels based on the patient’s needs. Custom synthesis allows pharmacological or chemical molecules to be tailored to the demands of the consumer. Finally, FDA officials inspect drug development laboratories to guarantee the purity and quality of the medications.
Infographic provided by The Emmes Company, a clinical research organization.